Maximum residue levels for mandipropamid
- Food safety
- Pesticide MRLs
Summary
The EU has increased the maximum residue levels (MRLs) for mandipropamid on gherkins, pumpkins, watermelons, and radish leaves.
This follows MRL increases for mandipropamid on papayas in February 2024 and for citrus fruits, kumquats, and animal fats in June 2023.
EU aligns MRLs for mandipropamid on cucumbers, gherkins and courgettes with Codex standards
Commission Regulation (EU) 2024/2633 of 8 October 2024 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azoxystrobin, famoxadone, flutriafol, mandipropamid and mefentrifluconazole in or on certain products
Commission Regulation (EU) 2024/344 of 22 January 2024 amending and correcting Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for mandipropamid in or on certain products
Commission Regulation (EU) 2023/1069 of 1 June 2023 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bixafen, cyprodinil, fenhexamid, fenpicoxamid, fenpyroximate, flutianil, isoxaflutole, mandipropamid, methoxyfenozide, and spinetoram in or on certain products
Update
The EU has increased the maximum residue levels (MRLs) for mandipropamid on gherkins, pumpkins, watermelons, and radish leaves.
This follows MRL increases for mandipropamid on papayas in February 2024 and for citrus fruits, kumquats, and animal fats in June 2023.
Impacted Products
Gherkins, pumpkins, watermelons, radish leaves, grapefruits, oranges, lemons, limes, mandarins, kumquats, papayas, fat from swine, cattle, sheep, goats, horses, other farmed animals
What is changing?
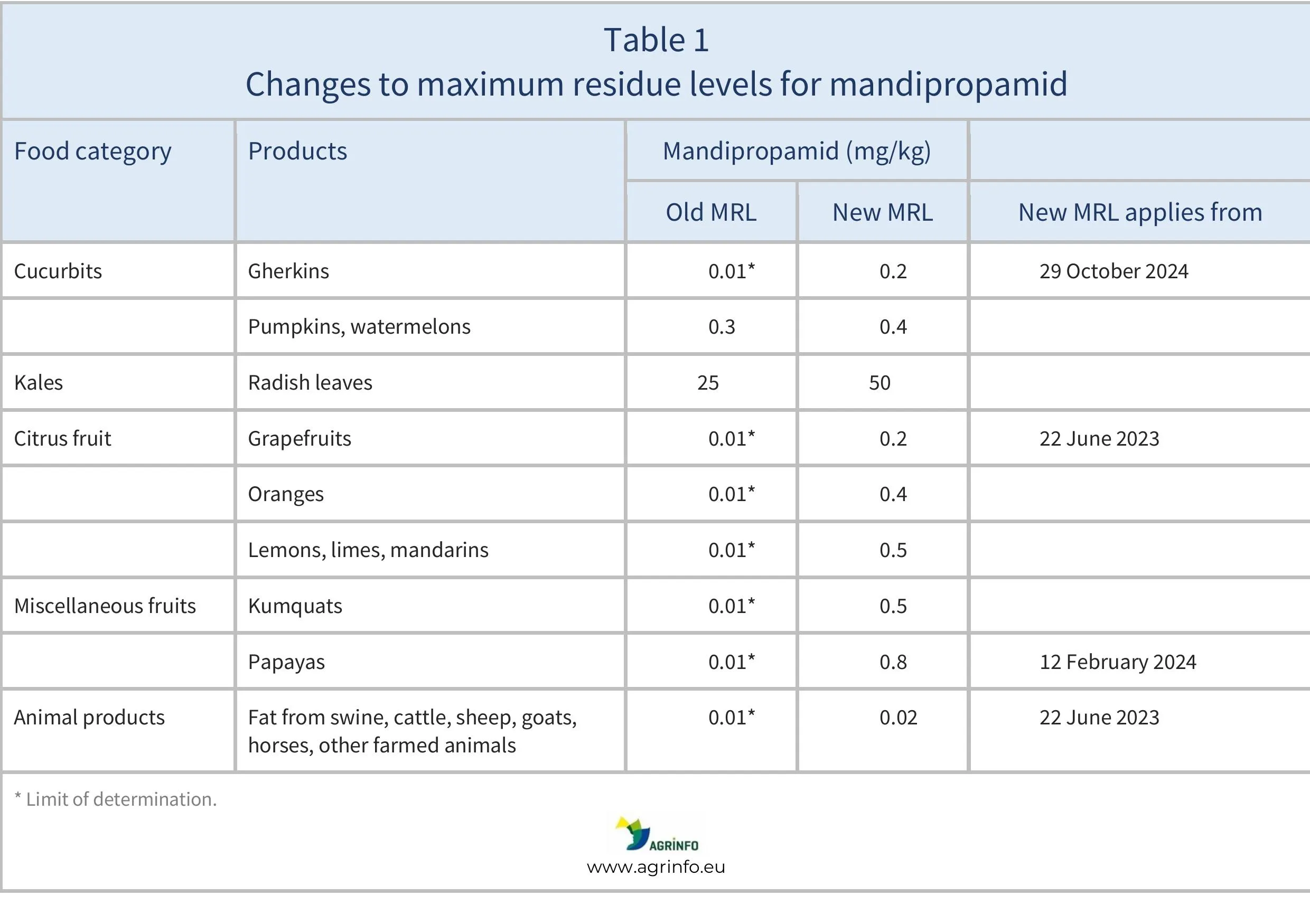
The EU has increased the MRLs for mandipropamid on gherkins from 0.01 to 0.2 mg/kg, and on pumpkins and watermelons from 0.3 to 0.4 mg/kg. Radish leaves are classified under the category “kales”. However, while the MRL for kales in general is 25 mg/kg, the MRL for radish leaves is raised from 25 to 50 mg/kg. There were MRL increases for other products in 2023 and early 2024. All changes are summarised in Table 1.
Why?
On 2 December 2023, the Codex Alimentarius Commission adopted new Codex maximum residue limits (CXLs) for mandipropamid on gherkins, pumpkins, and watermelons, for which the European Food Safety Authority (EFSA 2023a) did not identify risks to consumers in the EU.
Following a request for modification of the MRL for mandipropamid on radish leaves, EFSA (2023b) concluded that the proposed MRL is acceptable for consumer safety. The EU received an application for an import tolerance MRL with reference to use of mandipropamid on papayas in Brazil. On the basis of an evaluation by EFSA (2023c), it was concluded that there were no risks to consumers at the proposed level. The MRL increases in 2023 reflected new Codex MRLs (CXLs) that were adopted and subsequently evaluated as safe by EFSA (2022).
Timeline
The new MRLs on gherkins, pumpkins, watermelons, and radish leaves apply from 29 October 2024.
The application dates for earlier MRL changes can be found in Table 1.
Background
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
For further information on the setting of import tolerances, see Pesticide residue import tolerance MRLs explained.
Resources
EFSA (2022) Scientific support for preparing an EU position in the 53rd session of the codex committee on pesticide residues (CCPR). EFSA Journal, 20(9): 7521.
EFSA (2023a) Scientific support for preparing an EU position in the 54th Session of the Codex Committee on Pesticide Residues (CCPR) . EFSA Journal, 21(8): 8111.
EFSA (2023b) Modification of the existing maximum residue level for mandipropamid in radish leaves. EFSA Journal, 21: e8421.
EFSA (2023c) Setting of import tolerances for mandipropamid in papayas. EFSA Journal, 21(1): 7741.
Sources
Commission Regulation (EU) 2024/2633 as regards maximum residue levels for azoxystrobin, famoxadone, flutriafol, mandipropamid and mefentrifluconazole in or on certain products
Commission Regulation (EU) 2024/344 as regards maximum residue levels for mandipropamid in or on certain products
Commission Regulation (EU) 2023/1069 as regards maximum residue levels for bixafen, cyprodinil, fenhexamid, fenpicoxamid, fenpyroximate, flutianil, isoxaflutole, mandipropamid, methoxyfenozide, and spinetoram in or on certain products
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU aligns MRLs for mandipropamid on cucumbers, gherkins and courgettes with Codex standards
Commission Regulation (EU) 2024/2633 as regards maximum residue levels for azoxystrobin, famoxadone, flutriafol, mandipropamid and mefentrifluconazole in or on certain products
Commission Regulation (EU) 2024/344 as regards maximum residue levels for mandipropamid in or on certain products
Commission Regulation (EU) 2023/1069 as regards maximum residue levels for bixafen, cyprodinil, fenhexamid, fenpicoxamid, fenpyroximate, flutianil, isoxaflutole, mandipropamid, methoxyfenozide, and spinetoram in or on certain products
What is changing and why?
The EU has raised the maximum residue level (MRL) on gherkins from 0.01 to 0.2 mg/kg; on pumpkins and watermelons from 0.3 to 0.4 mg/kg based on new Codex MRLs (CXLs); and the MRL on radish leaves from 25 to 50 mg/kg, following a request for modification of the MRL and EFSA’s (2023b) evaluation that the requested modification is safe.
This follows MRL increases for mandipropamid on a number of products in 2023 and early 2024 as summarised in Table 1.
Timeline
The new MRLs on gherkins, pumpkins, watermelons, and radish leaves apply from 29 October 2024.
Application dates for other MRL increases are summarised in Table 1.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.