Maximum residue levels for nicotine
- Pesticide MRLs
Summary
The EU has amended the limit of determination (LOD) for nicotine on coffee from 0.01 to 0.05 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
EU amends MRLs for nicotine on coffee
Commission Regulation (EU) 2025/115 of 21 January 2025 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine in or on certain products
Update
The EU has amended the limit of determination (LOD) for nicotine on coffee from 0.01 to 0.05 mg/kg. (The LOD is the lowest level that can be detected using the most modern and reliable analytical methods.)
Impacted Products
Rose hips, herbs, edible flowers, wild fungi, teas, herbal infusions, spices, coffee
What is changing?
The European Union (EU) has amended the LOD for nicotine on coffee from 0.01 to 0.05 mg/kg.
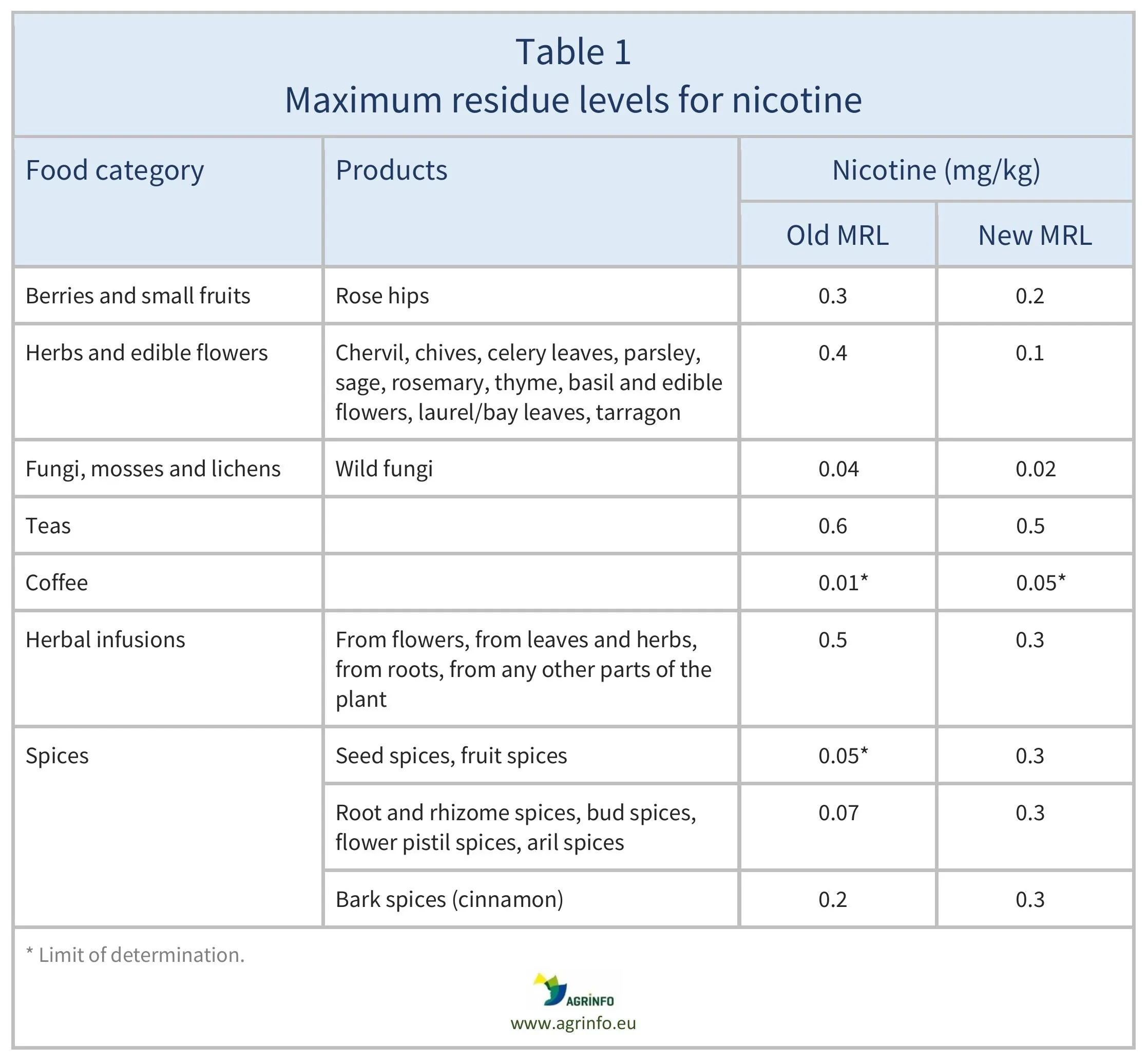
Previously, a targeted risk assessment of maximum residue levels (MRLs) for nicotine carried out by EFSA (2023) concluded that a nicotine MRL of 0.3 mg/kg on spices (seed, fruit, root, bark, bud, flower pistil, and aril spices) is safe for consumers. So for all spices the European Commission has reinstated the MRL of 0.3 mg/kg that applied before 15 September 2023.
Other nicotine MRL changes introduced in 2023 are set out in Table 1. The MRLs for all other products not listed in Table 1 are set to the LOD.
Why?
The MRL for nicotine in coffee beans has been changed to a temporary level of 0.05 mg/kg, based on monitoring data and a recommendation from the EU Reference Laboratory to account for residues from non-pesticide sources.
Following a review of the temporary MRLs in place since 2011, new Regulations were introduced in 2023 to lower the EU MRLs for nicotine (Regulations 2023/377 and 2023/1536).
In the case of capers, there were concerns that the temporary MRL of 4 mg/kg could pose an acute risk to consumers (according to calculations performed with EFSA's Pesticide Residue Intake Model, PRIMo rev. 3.1).
A potential acute risk for consumers was also identified for the temporary MRLs for nicotine in rose hips and teas, taking into account recent food consumption data for these products representative for European consumers.
In the case of spices (seed, fruit, root, bark, bud, flower pistil, and aril spices), new monitoring data were provided by non-EU exporting countries and food business operators, and a review was requested. A risk assessment carried out by EFSA (2023) concluded that the original nicotine MRL is safe for consumers, and the European Commission subsequently reinstated for all spices the MRL of 0.3 mg/kg.
Timeline
The new MRL for nicotine on coffee applies from 11 February 2025.
The revised MRL for nicotine on spices entered into force on 26 February 2024.
The MRLs for nicotine approved in 2023 have applied since 15 September 2023.
Recommended Actions
Suppliers to the EU market of rose hips, herbs and edible flowers, wild fungi, teas, herbal infusions, and spices should maintain, and where necessary increase, systematic monitoring of nicotine residues.
Monitoring data for nicotine residues on rose hips, herbs, edible flowers, teas, herbal infusions, and spices must be submitted to the EU by 22 February 2030 to be considered during the MRL review process. For fresh wild fungi, the deadline for submitting monitoring data is 30 June 2025, and for dried wild mushrooms, it is 25 July 2029.
EFSA (2024) gives detailed guidance on how to submit data to the European Food Safety Authority.
It is also important to note that nicotine is not approved for use as an insecticide in the EU, and should not be applied to crops that are intended for export to the EU due to the likelihood of exceeding permitted residue levels.
Background
In 2009, the EU authorisation for the use of nicotine as an active substance in plant protection products was withdrawn. No specific MRLs for nicotine were set, and the default MRL of 0.01 mg/kg was applied to all products.
In 2009 and 2011, the European Commission asked EFSA to provide advice on the setting of temporary MRLs for nicotine for a number of commodities in which residue levels greater than the default MRL were repeatedly identified during controls by food business operators and/or national competent authorities (EFSA 2009, 2011). Based on the EFSA assessment, specific temporary MRLs were set in Annex IIIA of Regulation (EC) No 396/2005 for nicotine in wild fungi (Commission Regulation (EU) 765/2010); and for nicotine in rose hips, herbs and edible flowers, teas, herbal infusions, and spices (Commission Regulation (EU) 812/2011).
The source of residues in these commodities has not been established. Contamination during harvest, drying, storage, or transport is considered possible. Nicotine also occurs naturally as the main alkaloid in tobacco and related species, and is found in low concentrations in certain other crops. In the absence of scientific evidence on the source, the Commission decided to review the temporary MRLs after 10 years in order to take into account any new information available. On the basis of this review, two new draft Regulations were introduced in 2022 referencing recent monitoring and food consumption data (EFSA 2022).
MRLs are set in accordance with the rules set out in Regulation 396/2005. For information on current MRLs for other substances, please consult the EU Pesticide Residues database.
Resources
EFSA (2009) Statement on the potential risks for public health due to the presence of nicotine in wild mushrooms. EFSA Journal, 7(5): 286.
EFSA (2011) Reasoned opinion on the setting of temporary MRLs for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs. EFSA Journal, 9(3): 2098.
EFSA (2022) Statement on the short‐term (acute) dietary risk assessment for the temporary maximum residue levels for nicotine in rose hips, teas and capers. EFSA Journal, 20(9): 7566.
EFSA (2023) Targeted risk assessment of maximum residue levels for nicotine in spices. EFSA Journal, 21(10): 8372.
EFSA (2024) Data collection. European Food Safety Authority.
Sources
Commission Regulation (EU) 2024/451 as regards maximum residue levels for nicotine in or on certain products
Commission Regulation (EU) 2023/1536 as regards maximum residue levels for nicotine in or on certain products
Commission Regulation (EU) 2023/377 as regards didecyldimethylammonium chloride (DDAC), flutriafol, metazachlor, nicotine, profenofos, quizalofop-P, sodium aluminium silicate, thiabendazole and triadimenol in or on certain products
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.
EU amends MRLs for nicotine on coffee
Commission Regulation (EU) 2025/115 as regards maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine in or on certain products
What is changing and why?
The European Union (EU) has amended the maximum residue level (MRL) for nicotine on coffee beans to a temporary level of 0.05 mg/kg, based on monitoring data and a recommendation from the EU Reference Laboratory to account for residues from non-pesticide sources.
Previously, after new data on nicotine became available, a risk assessment of MRLs for nicotine was carried out by the European Food Safety Authority (EFSA). EFSA concluded that for spices (seed, fruit, root, bark, bud, flower pistil, and aril spices) a nicotine MRL of 0.3 mg/kg is safe for consumers. So for all spices the European Commission has reinstated the MRL of 0.3 mg/kg.
Other changes to nicotine MRLs introduced in 2023 are shown in Table 1. The MRLs for all other products not listed in the table are set to the limit of determination (LOD, the lowest level that can be detected using the most modern and reliable analytical methods).
Actions
Suppliers to the EU market of rose hips, herbs and edible flowers, wild fungi, teas, herbal infusions, and spices should maintain – and where necessary increase – systematic monitoring of nicotine.
Monitoring data for nicotine residues on rose hips, herbs, edible flowers, teas, herbal infusions, and spices must be submitted to the EU by 22 February 2030 to be considered during the MRL review process. For fresh wild fungi, the deadline for submitting monitoring data is 30 June 2025, and for dried wild mushrooms, it is 25 July 2029.
EFSA’s Data collection webpage gives detailed guidance on how to submit data to the European Food Safety Authority.
Timeline
The new MRL for nicotine on coffee applies from 11 February 2025.
The revised MRL for nicotine on spices entered into force on 26 February 2024.
The MRLs for nicotine approved in 2023 have applied since 15 September 2023.
Disclaimer: Under no circumstances shall COLEAD be liable for any loss, damage, liability or expense incurred or suffered that is claimed to have resulted from the use of information available on this website or any link to external sites. The use of the website is at the user’s sole risk and responsibility. This information platform was created and maintained with the financial support of the European Union. Its contents do not, however, reflect the views of the European Union.